A the number of protons in the atom B the number of electrons in the atom C the number of neutrons in the atom D the number of protons electrons and neutrons in the atom E the net electrical charge of the atom. Therefore the answer is D.

Atomic Number Chemistry For Non Majors
There are 9 protons because the atomic number is always equal to the number of protons present.
. Atomic number describes about number of protonsIt is equals to number of protons. Determine the number of protons present. The atomic number of fluorine is 9.
Exists in orbit around the nucleus of an atom and carries a negative charge. A It would form two covalent bonds with other atoms. The atom has 20 protons.
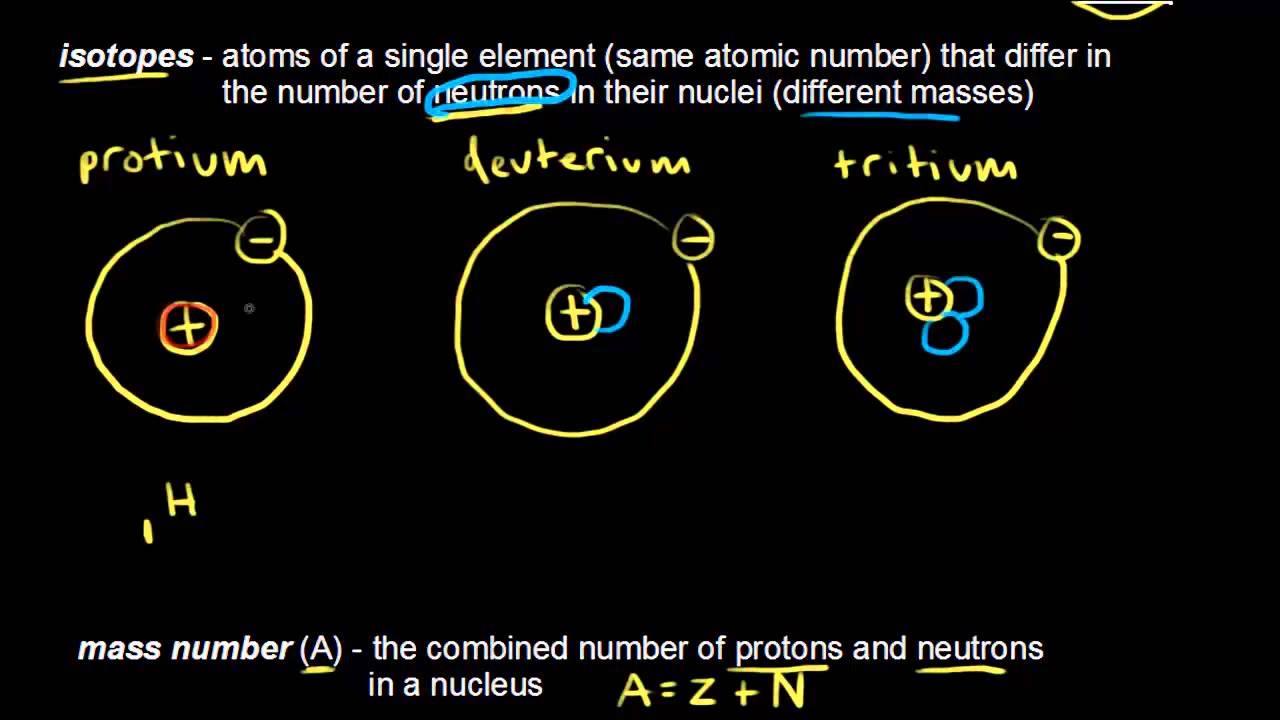
15 Typically nitrogen atoms are composed of electrons protons and neutrons. The combined number of protons and neutrons in an atom is called the atomic mass number. This is because some elements have a.
Which statement best describes an atom with an atomic number of 20. BThe number of protons is given by the mass number which increases by one moving from left to right across each row of the periodic table. How many neutrons does it have.
What is the mass number of a fluorine atom with 8 neutrons. An atom has an atomic number of 9 and a mass number of 19. The number of neutrons in an atom is equal to the difference between the atomic number and the atomic mass.
Mass number atomic number number of neutrons. The number of protons in the nucleus is called the atomic number. Determine the number of electrons present.
Fluorine is located in period 2 of the Periodic Table which implies that the 9th electron in fluorine is located on the second energy level. The atomic number represented by the letter Z of an element is the number of protons in the nucleus of each atom of that elementAn atom can be classified as a particular element based solely on its atomic number. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
The atom has 20 protons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electronsThe nucleus is composed of protons and neutronsTotal number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19. While the atomic number always stays the same some elements have atoms with different atomic mass numbers.
Determine the number of neutrons present. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. In this case it is 9 and yes it is F.
CThe number of protons is given by the atomic number which. However it is not an atom because it has 10 electrons so you have a fluoride ion F-. Different atomic forms of an element contain the same number of protons but a different number of neutrons.
The mass of an element. How many electrons are represented in the Lewis dot structure for aluminum Al. An atom of fluorine has an atomic number of 9 and an atomic mass of 19.
Question 9 Which statement is true. The atomic mass of an atom is equal to the number of neutrons in the nucleus The atomic number of an atom is equal to the number of neutrons in its nucleus The atomic weight of an atom is the sum of neutrons plus electrons The atomic weight of an atom is the sum of its protons and neutrons. A subatomic particle that carries no charge.
16_ 16 Which of the following best describes how an atom with atomic number 12 would behave in terms of forming bonds with other elements. The total electrical charge of the nucleus is therefore Ze where e elementary charge. Which of the following best describes the atomic number of an atom.
If you have mass number and atomic number subtract the atomic number from the mass number to get the number of neutrons. For example any atom with an atomic number of 8 its nucleus contains 8 protons is an oxygen atom and any atom with a different. 11 An atom with an atomic number of 9 and a mass number of 19 would have an atomic mass of approximately.
The smallest unit of a pure substance that consists of two or more atoms held together by a chemical bond. You know that fluorine has an atomic number equal to 9 which means that the 9th electron in an atom of fluorine is the last one added when constructing the neutral atom of fluorine. 14 ______ Athe number of neutrons in the atomBthe number of protons electrons and neutrons in the atom C the number of electrons in the atomD the number of protons in the atom.
The number of neutrons 19 - 9 10. You get the element by looking at the atomic number which is the number of protons. Carbon has an atomic number of 6 atomic number is the number of protons in the nucleus of an atom therefore carbon has 6 protons and since the number of electrons are equal to the number of protons in a neutral atom then the number of electrons in carbon atom is 6.
Helpful 0 Not Helpful 0 Add a Comment. The sum of the number of protons and electrons is 20. The atomic number of each element is unique.
Mass number is the sum of the protons and neutrons n0 of a particular isotope. Atomic number also called proton number refers to the. Fluorine is the element in question as its atomic number is 9.
Atomic Number Protons Electrons and Neutrons in Fluorine. 14 Which of the following best describes the atomic number of an atom. AThe number of protons is given by the atomic number which increases by one moving from left to right across each row of the periodic table.
A neutral atom of fluorine has a mass number of 1 9 and an atomic number of 9. The sum of the number of protons and neutrons is 20. The atom has 20 neutrons.
Solved Can You Create An Atom With Atomic Number Equal To 2 Course Hero

Bromine Periodic Table And Atomic Properties

0 Comments